AFLDS Mask Mandate Letter
This document was written by America's Frontline Doctors to address the issues with mandated mask wearing. All references in the letter are provided, and when possible PDF download links are also provided.
You can download the latest version of this document at their website using the links below.
America's Frontline Doctors Letter (original source):
https://americasfrontlinedoctors.org/wp-content/uploads/2021/07/6076ddcb96c12c1e6e611614_Mask-Public-Letter-Adult-1.pdf
America's Frontline Doctors page with additional mask documents:
https://americasfrontlinedoctors.org/legal/masks-the-law/
The Letter
Date: {Todays Date}
To Whom It May Concern:
I am sending you this notice, related to {ORGANIZATION NAME}’s mask policy. My findings raise significant concerns, both medically and legally, of the current mask policy in place. Masks are ineffective for the purpose claimed by the mandate, potentially harmful, and only authorized for use by an EUA.
Masks are ineffective and in many ways they harm.
It’s a myth that masks prevent viruses from spreading. The overall evidence is clear: Standard cloth and surgical masks offer next to no protection against virus-sized particles or small aerosols.(1) The size of a virus particle is much too small to be stopped by a surgical mask, cloth or bandanna. A single virion of SARS-CoV-2 is about 60-140 nanometers or 0.1 microns.(2) The pore size in a surgical mask is 200-1000x that size. Consider that the CDC website states, “surgical masks do not catch all harmful particles in smoke.”(29) And that the size of smoke particles in a wildfire are ~0.5 microns which is 5x the size of the SARS-CoV-2 virus! Wearing a mask to prevent catching SARS-CoV-2, or similarly sized influenza, is like throwing sand at a chain-link fence: it doesn’t work. There has been one large randomized controlled trial that specifically examined whether masks protect their wearers from the coronavirus. This study found mask wearing “did not reduce, at conventional levels of statistical significance, the incidence of Sars-Cov-2-infection.”(3)
Consider also, that the existence of more particles does not mean more virus. Research shows less virus does not mean less illness. Dr. Kevin Fennelly, a pulmonologist at the National Heart, Lung and Blood institute debunked the view that larger droplets are responsible for viral transmission. Fennelly wrote:
“current infection control policies are based on the premise that most respiratory infections are transmitted by large respiratory droplets- i.e., larger than 5 [microns] – produced by coughing and sneezing" …"This Viewpoint (article) suggests that infection control guidelines should be re-evaluated to account for the predominance of small particles within infectious aerosols.”(4)
Fennelly referenced a 1953 paper on anthrax that showed a single bacterial spore of about one micron was significantly more lethal than larger clumps of spores. (5) Exposure to one virus particle is theoretically enough to cause infection and subsequent disease. This is not an alarming thought - it simply means what it has always meant, that our immune system protects us continually all our life. (6)
There have been hundreds of mask studies related to influenza transmission done over several decades. It is a well-established fact that masks do not stop viruses. “Part of that evidence shows that cloth facemasks actually increase influenza-linked illness.”(7) Bacteria are 50x larger than virus particles.(8) As such, virus particles can enter through the mask pores, yet bacteria remain trapped inside of the mask, resulting in the mask-wearer continually exposed to the bacteria.
Related to the 1918-1919 influenza pandemic, there was almost universal agreement among experts, that deaths were virtually never caused by the influenza virus itself but resulted directly from severe secondary pneumonia caused by well-known bacterial “pneumopathogens” that colonized the upper respiratory tract.(9) Dr. Fauci and his National Institute of Health studied pandemics and epidemics and concluded, “the vast majority of influenza deaths resulted from secondary bacterial pneumonia.”(10)
All parties mandating the use of facemasks are not only willfully ignoring established science but are engaging in what amounts to a clinical experimental trial. This conclusion is reached by the fact that facemask use and Covid-19 incidence are being reported in scientific opinion pieces promoted by the CDC and others.(11) The fact is after reviewing ALL of the studies worldwide, the CDC found the use of facemasks "did not support a substantial effect on transmission of laboratory-confirmed influenza."(12)
Any intervention, especially one that is prophylactic, must cause fewer harms to the recipient than the infection. The cost-benefit of mandating an investigational face-covering with emerging safety issues is especially difficult to justify. Anthony Fauci was very clear that asymptomatic transmission was not a threat. He stated, “in all the history of respiratory-borne viruses of any type, asymptomatic transmission has never been the driver of outbreaks. The driver of outbreaks is always a symptomatic person.”(13)
Wearing respirators come(s) with a host of physiological and psychological burdens. These can interfere with task performances and reduce work efficiency. These burdens can even be severe enough to cause life-threatening conditions if not ameliorated.(14) Fifteen years ago, National Taiwan University Hospital concluded that the use of N-95 masks in healthcare workers caused them to experience hypoxemia, a low level of oxygen in the blood, and hypercapnia, an elevation in the blood's carbon dioxide levels.(15) Studies of simple surgical masks found significant reductions in blood oxygen as well. In one particular study, researchers measured blood oxygenation before and after surgeries in 53 surgeons. Researchers found the mask reduced the blood oxygen levels significantly, and the longer the duration of wearing the mask, the greater the drop in blood oxygen levels.(16)
Moreover, people with cancer will be at a further risk from hypoxia, as cancer cells grow best in a bodily environment that is low in oxygen. Low oxygen also promotes systemic inflammation which, in turn, promotes “the growth, invasion and spread of cancers.”(17) Repeated episodes of low oxygen, known as intermittent hypoxia, also “causes atherosclerosis” and hence increases “all cardiovascular events” such as heart attacks, as well as adverse cerebral events like stroke.(18)
Informed consent is required for investigational medical therapies.
Regardless of the lack of safety and efficacy behind the decision to require employees to wear a mask, it is illegal to mandate EUA approved investigational medical therapies without informed consent. Mask use for viral transmission prevention is authorized for Emergency Use only.(19) Emergency Use Authorization by the FDA, means “the products are investigational and experimental” only.(20) The statute granting the FDA the power to authorize a medical product of emergency use requires that the person being administered the unapproved product be advised of his or her right to refuse administration of the product.(21) This statute further recognizes the well-settled doctrine that medical experiments, or “clinical research,” may not be performed on human subjects without the express, informed consent of the individual receiving treatment.(22)
The right to avoid the imposition of human experimentation is fundamental, rooted in the Nuremberg Code of 1947, has been ratified by the 1964 Declaration of Helsinki, and further codified in the United States Code of Federal Regulations. In addition to the Unites States regarding itself as bound by these provisions, these principles were adopted by the FDA in its regulations requiring the informed consent of human subjects for medical research.(23) The law is very clear; It is unlawful to conduct medical research (even in the case of emergency), unless steps taken to … secure informed consent of all participants.(24)
Furthermore, by requiring employees to wear a mask, you are promoting the idea that the mask can prevent or treat a disease, which is an illegal deceptive practice. It is unlawful to advertise that a product or service can prevent…disease unless you possess competent and reliable scientific evidence… substantiating that the claims are true.(25)
The FDA EUA for surgical and/or cloth masks explicitly states, “the labeling must not state or imply… that the [mask] is intended for antimicrobial or antiviral protection or related, or for use such as infection prevention or reduction.”(26) As you can see from the image below, masks do not claim to keep out viruses.
Illegally mandating an investigational medical therapy generates liability.
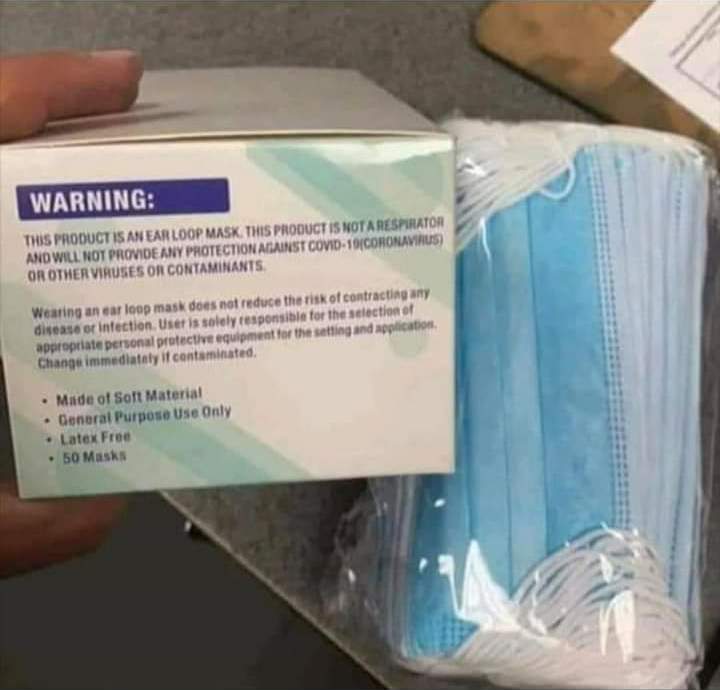
There are proven microbial challenges as well as breathing difficulties that are created and exacerbated by extended mask-wearing.
Requiring employees to wear a mask sets the stage for contracting any infection, including COVID-19, and making the consequences of that infection much graver. In essence, a mask may very well put us at an increased risk of infection, and if so, having a far worse outcome.(27)
The fact that mask wearing presents a severe risk of harm to the wearer should – standing alone – not be required for employees, particularly given that we are not ill and have done nothing wrong that would warrant an infringement of our constitutional rights and bodily autonomy. Promoting use of a non-FDA approved, Emergency Use Authorized mask, is unwarranted and illegal. This mandate is in direct conflict with Section 360bbb-3e(1)(A)(ii)(I-III) (28), which requires the wearer to be informed of the option to refuse the wearing of such “device.” Misrepresenting the use of a mask as being intended for antimicrobial or antiviral protection, and/or misrepresenting masks for use as infection prevention or reduction is a deceptive practice under the FTC. It is clear, there is no waiver of liability under deceptive practices, even under a state of emergency. As such, forcing employees to wear masks, or similarly forcing use any other non-FDA approved medical product without the wearer’s consent, is illegal and immoral.
This letter serves as official notice that I do not consent to being forced to wear a mask. I will not fail to take the maximum action permissible under the law against your organization, and against you personally. Accordingly, I urge you to comply with Federal and State law, and advise employees they have a right to refuse or wear a mask as a measure to prevent or reduce infection from Covid-19. Any other course of action is contrary to the law. I am willing to testify as to the veracity of the contents in this document. Please confirm no further pressure will be exerted upon me to follow this illegal mask mandate, and that I will not face any retaliatory disciplinary action.
Sincerely,
{SIGNATURE}
{PRINTED NAME}
References
PDF files were collected and added to this document for each reference where available.
https://www.amazon.com/Unreported-Truths-About-Covid-19-Lockdowns/dp/1953039073/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2218003/
DOWNLOAD PDF
https://books.google.com/books?hl=en&lr=&id=UYMBXV4hJQQC&oi=fnd&pg=PA21&ots=aCHUTuHe6L&sig=MNN0-5yBmNa8p1OUvLSM80MZHYU#v=onepage&q&f=true
The link above says the page is archived. Below is what appears to be the updated page.
https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/about-face-coverings.html
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7181938/
DOWNLOAD PDF
https://www.youtube.com/watch?v=X1orSO094uY
https://jbioleng.biomedcentral.com/articles/10.1186/s13036-016-0025-4
DOWNLOAD PDF
https://www.clinicaltrials.gov/ct2/show/record/NCT00173017
DOWNLOAD PDF
https://pubmed.ncbi.nlm.nih.gov/18500410/
DOWNLOAD PDF
1. https://www.cell.com/cancer-cell/fulltext/S1535-6108(04)00244-2
DOWNLOAD PDF
2. https://surgicalneurologyint.com/surgicalint-articles/immunoexcitatory-mechanisms-in-glioma-proliferation-invasion-and-occasional-metastasis/
DOWNLOAD PDF
https://pubmed.ncbi.nlm.nih.gov/17332479/
DOWNLOAD PDF
https://www.fda.gov/media/137121/download
DOWNLOAD PDF
FDA Letter of Authorization (EUA) for Surgical Masks:
https://www.fda.gov/media/140894/download
DOWNLOAD PDF
https://uscode.house.gov/view.xhtml?req=(title:21%20section:360bbb-3a%20edition:prelim)
21 CFR 812.20(b)(11)
https://www.law.cornell.edu/cfr/text/21/812.20
DOWNLOAD PDF
https://uscode.house.gov/view.xhtml?req=(title:21%20section:360bbb-3a%20edition:prelim)
https://www.law.cornell.edu/cfr/text/21/50.20
21 C.F.R. § 50.23
https://www.law.cornell.edu/cfr/text/21/50.23
21 C.F.R. §50.20
https://www.law.cornell.edu/cfr/text/21/50.20
21 C.F.R. § 50.24
https://www.law.cornell.edu/cfr/text/21/50.24
FTC Act, 15 U.S. Code § 45 (a)(1)
https://www.law.cornell.edu/uscode/text/15/45
FDA Letter of Authorization (EUA) for Face Masks:
https://www.fda.gov/media/137121/download
DOWNLOAD PDF
https://pubmed.ncbi.nlm.nih.gov/26179900/
DOWNLOAD PDF
See also: Westendorf AM et al. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell Physiol Biochem 2017;41:1271-84.
https://pubmed.ncbi.nlm.nih.gov/28278498/
DOWNLOAD PDF
See further: Sceneay J et al. Hypoxia-driven immunosuppression contributes to the pre-metastatic niche. Oncoimmunology 2013;2:1 e22355.
https://www.tandfonline.com/doi/full/10.4161/onci.22355
DOWNLOAD PDF
https://www.cdc.gov/disasters/covid-19/wildfire_smoke_covid-19.html
Additional Notes
Additional notes included below were written by Edward A Scharf.
https://www.thelanced.com/journals.lanres/article/PIIS2213-2600(20)30323-4/fulltext
I found the original document and corrected the URL in this document.
I searched for the indented quote ion the original letter:
“current infection control policies are based on the premise that most respiratory infections are transmitted by large respiratory droplets- i.e., larger than 5 [microns] – produced by coughing and sneezing, …Unfortunately, that premise is wrong.”
The first part of the Fennelly quote was found on page 914 of the pdf document. The second part of the quote "Unfortunately, that premise is wrong" is NOT present in the document anywhere. Not even parts of it are presentHowever in the discussion on page 921 of the document it says, "This Viewpoint (article) suggests that infection control guidelines should be re-evaluated to account for the predominance of small particles within infectious aerosols.” So I entered that in my letter above. The total quote becomes:
“current infection control policies are based on the premise that most respiratory infections are transmitted by large respiratory droplets- i.e., larger than 5 [microns] – produced by coughing and sneezing" …"This Viewpoint (article) suggests that infection control guidelines should be re-evaluated to account for the predominance of small particles within infectious aerosols.”
Druett HA, Henderson DW, Packman L, Peacock S. Studies on respiratory infection. I. The influence of particle size on respiratory infection with anthrax spores. J Hyg (Lond) 1953; 51: 359–71
I replaced the letter reference with this one (Druett).
Conclusions: This study is the first RCT of cloth masks, and the results caution against the use of cloth masks. This is an important finding to inform occupational health and safety. Moisture retention, reuse of cloth masks and poor filtration may result in increased risk of infection. Further research is needed to inform the widespread use of cloth masks globally. However, as a precautionary measure, cloth masks should not be recommended for HCWs, particularly in high-risk situations, and guidelines need to be updated.
"Although mechanistic studies support the potential effect of hand hygiene or face masks, evidence from 14 randomized controlled trials of these measures did not support a substantial effect on transmission of laboratory-confirmed influenza."
"There were 3 influenza pandemics in the 20th century, and there has been 1 so far in the 21st century. Local, national and international health authorities regularly update their plans for mitigating the next influenza pandemic in light of the latest available evidence on the effectiveness of nonpharmaceutical personal protective measures and environmental hygiene measures in non-healthcare settings and discuss their potential inclusion in pandemic plans. Although mechanistic studies support the potential effect of hand hygiene or face masks, evidence from 14 randomized controlled trials of these measures did not support a substantial effect on transmission of laboratory confirmed influenza. We similarly found limited evidence on the effectiveness of improved hygiene and environmental cleaning. We identified several major knowledge gaps requiring further research, most fundamentally an improved characterization of the modes of person-to-person transmission."
I replace the original quote in the letter, "no reduction in viral transmission with the use of face masks" with the following directly from the source --
the use of facemasks "did not support a substantial effect on transmission of laboratory-confirmed influenza."
The first EUA letter, issued April 24, 2020, states -- "Mask use for viral transmission prevention is authorized for Emergency Use only." HOWEVER the Emergency Use Authorization specifically states:
"Surgical masks are not covered within the scope of this authorization. Surgical masks are masks that cover the user’s nose and mouth and provide a physical barrier to fluids and particulate materials and are regulated under 21 CFR 878.4040 as class II devices requiring premarket notification. Additionally, these masks meet certain fluid barrier protection standards and Class I or Class II flammability tests. More information on the distinction is provided in FDA guidance, titled “Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency” available at https://www.fda.gov/media/136449/download."
I interpret that statement to mean that ONLY cloth facemasks are covered in the April 24 letter.
The second EUA, issued August 5, 2020, covers "surgical" masks specifically.
Also see: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/personal-protective-equipment-euas#appendixasurgicalmasks
https://www.fda.gov/medical-devices/investigational-device-exemption-ide/ide-informed-consent#ide
Important: Keep in mind that the "investigational device" does NOT apply to "surgical masks", but only to cloth facemasks. See ref 19 above. Geesh.
Here is the actual law in the Federal Register that requires informed consent in the case of investigational devices. 21 CFR 812.20(b)(11). (Cornell Law seems to be much easier to read than the actual Code of Federal Regulations (CFR))
https://www.law.cornell.edu/cfr/text/21/812.20
The text reads:
"(11) Copies of all forms and informational materials to be provided to subjects to obtain informed consent."
Except as provided in § 50.23 and 50.24, no investigator may involve a human being as a subject in research covered by these regulations unless the investigator has obtained the legally effective informed consent of the subject or the subject's legally authorized representative. An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence. The information that is given to the subject or the representative shall be in a language understandable to the subject or the representative. No informed consent, whether oral or written, may include any exculpatory language through which the subject or the representative is made to waive or appear to waive any of the subject's legal rights, or releases or appears to release the investigator, the sponsor, the institution, or it's agents from liability for negligence.
[46 FR 8951, Jan. 27, 1981, as ammended at 64 FR 10942, Mar. 8, 1999]
After a cursory search, I did find FTC Act, 15 U.S. Code § 45 (a)(1) which describes unfair and deceptive practices in commerce as unlawful
https://www.law.cornell.edu/uscode/text/15/45
It says:
(a) Declaration of unlawfulness; power to prohibit unfair practices; inapplicability to foreign trade (1) Unfair methods of competition in or affecting commerce, and unfair or deceptive acts or practices in or affecting commerce, are hereby declared unlawful.
(e) Conditions of Authorization (1) Unapproved Product (A) Required conditions With respect to the emergency use of an unapproved product, the Secretary, to the extent practicable given the applicable circumstances described in subsection (b)(1), shall, for a person who carries out any activity for which the authorization is issued, establish such conditions on an authorization under this section as the Secretary finds necessary or appropriate to protect the public health, including the following: (ii) Appropriate conditions designed to ensure that individuals to whom the product is administered are informed— (I) that the Secretary has authorized the emergency use of the product; (II) of the significant known and potential benefits and risks of such use, and of the extent to which such benefits and risks are unknown; and (III) of the option to accept or refuse administration of the product, of the consequences, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.